Lab Report Oxidation Of Alcohol . The experiment uses a chemical method based. write an equation to represent the oxidation of an alcohol. in this experiment, the alcohol content (% ethanol by volume) will be determined. you should be able to write equations for the oxidation of primary and secondary alcohols. primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. Identify the reagents that may be used to oxidize a given alcohol. form ethanal (acetaldehyde), and with further oxidation, ethanoic acid (acetic acid), ethanol is oxidised by acidified sodium dichromate in a test tube. write up a formal report as outlined in the lab report guidelines: You will be setting up. This reaction is used to. this page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. You should know that carboxylic acids are weak acids and that they.
from www.chegg.com
You will be setting up. write an equation to represent the oxidation of an alcohol. form ethanal (acetaldehyde), and with further oxidation, ethanoic acid (acetic acid), ethanol is oxidised by acidified sodium dichromate in a test tube. primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. in this experiment, the alcohol content (% ethanol by volume) will be determined. You should know that carboxylic acids are weak acids and that they. This reaction is used to. this page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. The experiment uses a chemical method based. write up a formal report as outlined in the lab report guidelines:
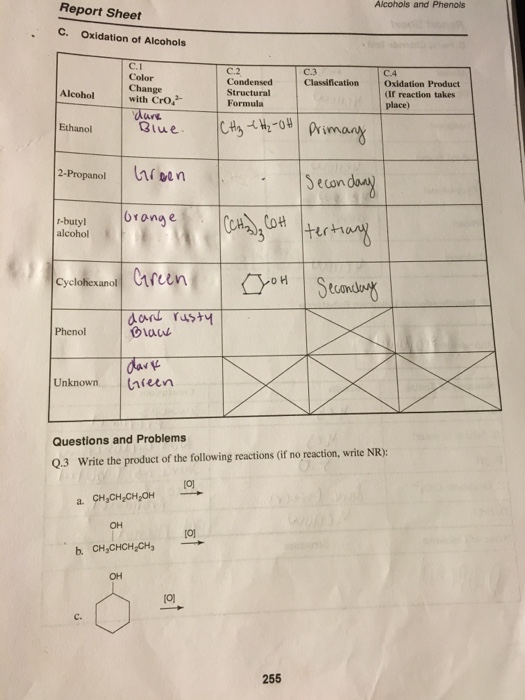
Solved Report Sheet Alcohols And Phenols C. Oxidation Of
Lab Report Oxidation Of Alcohol Identify the reagents that may be used to oxidize a given alcohol. this page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. The experiment uses a chemical method based. write up a formal report as outlined in the lab report guidelines: form ethanal (acetaldehyde), and with further oxidation, ethanoic acid (acetic acid), ethanol is oxidised by acidified sodium dichromate in a test tube. in this experiment, the alcohol content (% ethanol by volume) will be determined. write an equation to represent the oxidation of an alcohol. primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. This reaction is used to. Identify the reagents that may be used to oxidize a given alcohol. You will be setting up. you should be able to write equations for the oxidation of primary and secondary alcohols. You should know that carboxylic acids are weak acids and that they.
From www.chegg.com
Solved Report Sheet Alcohols And Phenols C. Oxidation Of Lab Report Oxidation Of Alcohol This reaction is used to. primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. form ethanal (acetaldehyde), and with further oxidation, ethanoic acid (acetic acid), ethanol is oxidised by acidified sodium dichromate in a test tube. Identify the reagents that may be used to oxidize a given alcohol. You should know that. Lab Report Oxidation Of Alcohol.
From studylib.net
EXPERIMENT 11 OXIDATION OF ALCOHOLS Lab Report Oxidation Of Alcohol write up a formal report as outlined in the lab report guidelines: primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. write an equation to represent the oxidation of an alcohol. this page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. You should know. Lab Report Oxidation Of Alcohol.
From www.studocu.com
LAB 1 Oxidation of an Unknown Alcohol Identification of an Unkown Lab Report Oxidation Of Alcohol write up a formal report as outlined in the lab report guidelines: The experiment uses a chemical method based. primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. You should know that carboxylic acids are weak acids and that they. form ethanal (acetaldehyde), and with further oxidation, ethanoic acid (acetic acid),. Lab Report Oxidation Of Alcohol.
From www.studocu.com
Lab Report 2 Oxidation of an Alcohol (Oxidation of Cyclododecanol Lab Report Oxidation Of Alcohol Identify the reagents that may be used to oxidize a given alcohol. The experiment uses a chemical method based. in this experiment, the alcohol content (% ethanol by volume) will be determined. This reaction is used to. you should be able to write equations for the oxidation of primary and secondary alcohols. write an equation to represent. Lab Report Oxidation Of Alcohol.
From www.masterorganicchemistry.com
Alcohol Oxidation "Strong" & "Weak" Oxidants Master Organic Chemistry Lab Report Oxidation Of Alcohol write an equation to represent the oxidation of an alcohol. form ethanal (acetaldehyde), and with further oxidation, ethanoic acid (acetic acid), ethanol is oxidised by acidified sodium dichromate in a test tube. The experiment uses a chemical method based. You should know that carboxylic acids are weak acids and that they. You will be setting up. in. Lab Report Oxidation Of Alcohol.
From www.youtube.com
Chemical Tests for Alcohols Lucas Test & Oxidation Tests // HSC Lab Report Oxidation Of Alcohol You will be setting up. in this experiment, the alcohol content (% ethanol by volume) will be determined. write up a formal report as outlined in the lab report guidelines: You should know that carboxylic acids are weak acids and that they. This reaction is used to. The experiment uses a chemical method based. this page looks. Lab Report Oxidation Of Alcohol.
From www.researchgate.net
Alcohol oxidation conditions. a Alcohols oxidation with oxidant. b Lab Report Oxidation Of Alcohol You should know that carboxylic acids are weak acids and that they. you should be able to write equations for the oxidation of primary and secondary alcohols. write an equation to represent the oxidation of an alcohol. The experiment uses a chemical method based. this page looks at the oxidation of alcohols using acidified sodium or potassium. Lab Report Oxidation Of Alcohol.
From www.chemistrysteps.com
Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps Lab Report Oxidation Of Alcohol primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. You should know that carboxylic acids are weak acids and that they. write an equation to represent the oxidation of an alcohol. You will be setting up. This reaction is used to. in this experiment, the alcohol content (% ethanol by volume). Lab Report Oxidation Of Alcohol.
From www.studocu.com
Lab 3Oxidation of an Alcohol EXPERIMENT 5 Oxidation of Alcohols Lab Report Oxidation Of Alcohol primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. write an equation to represent the oxidation of an alcohol. you should be able to write equations for the oxidation of primary and secondary alcohols. This reaction is used to. this page looks at the oxidation of alcohols using acidified sodium. Lab Report Oxidation Of Alcohol.
From studylib.net
31 EXPERIMENT 3 Oxidation of Alcohols Solid Lab Report Oxidation Of Alcohol primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. form ethanal (acetaldehyde), and with further oxidation, ethanoic acid (acetic acid), ethanol is oxidised by acidified sodium dichromate in a test tube. in this experiment, the alcohol content (% ethanol by volume) will be determined. This reaction is used to. write. Lab Report Oxidation Of Alcohol.
From www.tes.com
Oxidation of Alcohols AS Chemistry OCR Teaching Resources Lab Report Oxidation Of Alcohol You will be setting up. The experiment uses a chemical method based. you should be able to write equations for the oxidation of primary and secondary alcohols. You should know that carboxylic acids are weak acids and that they. write an equation to represent the oxidation of an alcohol. form ethanal (acetaldehyde), and with further oxidation, ethanoic. Lab Report Oxidation Of Alcohol.
From www.academia.edu
(DOC) Oxidation of a Secondary Alcohol Cyclohexanone from Cyclohexanol Lab Report Oxidation Of Alcohol Identify the reagents that may be used to oxidize a given alcohol. primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. in this experiment, the alcohol content (% ethanol by volume) will be determined. The experiment uses a chemical method based. You will be setting up. This reaction is used to. . Lab Report Oxidation Of Alcohol.
From www.studocu.com
Lab Report 9 Oxidation of Unknown Alcohols with KMnO4/CuSO4 under Lab Report Oxidation Of Alcohol in this experiment, the alcohol content (% ethanol by volume) will be determined. You should know that carboxylic acids are weak acids and that they. form ethanal (acetaldehyde), and with further oxidation, ethanoic acid (acetic acid), ethanol is oxidised by acidified sodium dichromate in a test tube. You will be setting up. you should be able to. Lab Report Oxidation Of Alcohol.
From graduateway.com
⇉Oxidation Lab Report Essay Example GraduateWay Lab Report Oxidation Of Alcohol The experiment uses a chemical method based. Identify the reagents that may be used to oxidize a given alcohol. This reaction is used to. You will be setting up. this page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. write up a formal report as outlined in the lab report guidelines: . Lab Report Oxidation Of Alcohol.
From www.pinterest.jp
Today’s post is a quick one for the chemistry students, with a look at Lab Report Oxidation Of Alcohol primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. write up a formal report as outlined in the lab report guidelines: this page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. You should know that carboxylic acids are weak acids and that they. The experiment. Lab Report Oxidation Of Alcohol.
From www.studocu.com
Orgo Lab 12 Lab Report 12 Experiment 33B Oxidation of an Alcohol Lab Report Oxidation Of Alcohol in this experiment, the alcohol content (% ethanol by volume) will be determined. You should know that carboxylic acids are weak acids and that they. write up a formal report as outlined in the lab report guidelines: You will be setting up. this page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi). Lab Report Oxidation Of Alcohol.
From www.studocu.com
Experiment 1 Oxidation of Unknown Alcohol Identification of Unknown Lab Report Oxidation Of Alcohol You should know that carboxylic acids are weak acids and that they. The experiment uses a chemical method based. You will be setting up. primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the reagents used. write up a formal report as outlined in the lab report guidelines: This reaction is used to. form. Lab Report Oxidation Of Alcohol.
From studylib.net
EXPERIMENT 11 OXIDATION OF ALCOHOLS Lab Report Oxidation Of Alcohol The experiment uses a chemical method based. in this experiment, the alcohol content (% ethanol by volume) will be determined. write an equation to represent the oxidation of an alcohol. This reaction is used to. form ethanal (acetaldehyde), and with further oxidation, ethanoic acid (acetic acid), ethanol is oxidised by acidified sodium dichromate in a test tube.. Lab Report Oxidation Of Alcohol.